The Centers for Disease Control and Prevention (CDC) has been compelled to release over 780,000 reports detailing adverse events post-COVID-19 vaccination. These reports, initially withheld from the public eye, reveal a range of serious health issues following vaccination, including heart inflammation, miscarriages, seizures, and facial paralysis.
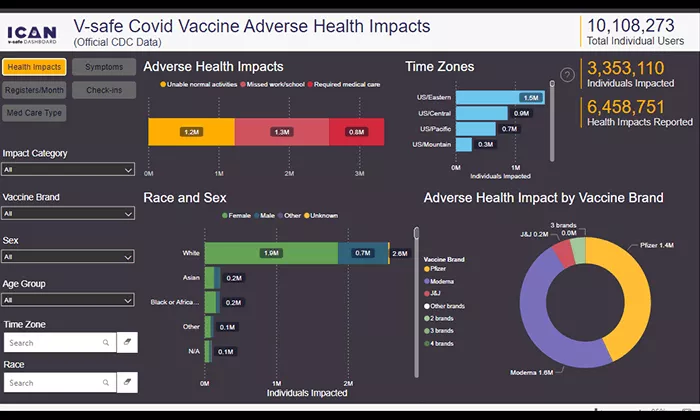
This table summarizes the key statistics from the CDC’s V-safe data on post-vaccination effects:
| Description | Number of Reports |
|---|---|
| Total individuals seeking medical attention | 782,900 |
| Individuals needing ER care/hospitalization | Included in above |
| People missing school, work, or normal activities | 2.5 million |
| Total symptoms reported | 71 million |
| Chills reported | 3.5 million |
| Swelling reported | 3.6 million |
| Joint pain reported | 4 million |
| Muscle/body aches reported | 7.8 million |
| Headache reported | 9.7 million |
| Fatigue reported | 12.7 million |
| General pain reported | 19.5 million |
| Symptoms of severe severity | 4.2 million |
| Reports for children under 2 years old | 13,000 |
| Symptoms for children under 2 | 33,000 |
This table provides a concise overview of the adverse effects reported following COVID-19 vaccination, as captured by the CDC’s V-safe program.
Legal Pressure and Public Disclosure
Under legal duress, the CDC disclosed these reports following a federal judge's order. The judge dismissed the CDC’s claims of the data release being burdensome, highlighting the necessity for transparency. This move has unveiled the vast number of health incidents post-vaccination, contradicting the CDC's previous assurances about vaccine safety.
Systematic Suppression of Information
The CDC’s long-standing refusal to make this data public, coupled with the revelation that nearly 8 percent of vaccine recipients experienced significant health impacts, raises serious questions about the agency's transparency and accountability. This data suppression is seen as a breach of public trust, undermining the CDC’s role as a guardian of public health.
Erosion of Public Confidence
The CDC's actions have significantly eroded trust in public health institutions. By prioritizing the concealment of crucial health data, the CDC has compromised its credibility and the public’s faith in vaccination campaigns. The delayed and reluctant release of information suggests a potential disregard for the severity of vaccine-related health issues and a failure to acknowledge the public’s right to informed decision-making. This lack of transparency not only fuels public skepticism but also exacerbates tensions surrounding vaccine passport policy issues, which have already proven to be deeply polarizing. Without clear and honest communication from the CDC, debates over these policies are increasingly mired in misinformation and mistrust. Rebuilding confidence will require the CDC to prioritize accountability and provide the public with comprehensive, unbiased data.
The revelation that hundreds of thousands of Americans sought medical attention post-COVID-19 vaccination underscores the gravity of the situation. CDC data, released after persistent legal pressure from the Informed Consent Action Network (ICAN), disclosed that approximately 782,900 individuals reported needing medical, emergency, or hospital care following their vaccination, with an additional 2.5 million people reporting significant disruptions to their daily lives.
These figures, derived from the CDC’s V-safe system—a smartphone-based monitoring tool—paint a stark picture of the vaccine's potential impact on public health. The sheer volume of reports, totaling about 71 million symptoms, most commonly being pain, fatigue, headaches, and chills, reflects a distressing trend that should have prompted immediate action from health authorities.
The CDC's delay in releasing this critical data, coupled with the fact that no new safety concerns were raised internally, further exemplifies a troubling lack of urgency and transparency, eroding public trust and raising serious questions about the management and communication of vaccine safety monitoring.
The CDC's handling of COVID-19 vaccine injury reports has been heavily criticized for undermining public trust and failing to uphold the standards of transparency and accountability expected of a leading public health agency.
The disclosure of these extensive reports of adverse effects following COVID-19 vaccination raises serious concerns about the CDC's transparency and responsiveness. Here are the key conclusions that can be drawn about the CDC in light of these facts:
- Delayed Transparency: The fact that the CDC withheld this significant volume of data until forced by legal action suggests a lack of proactive transparency. This delay in disclosure undermines the CDC's commitment to public health transparency and accountability, which are crucial for public trust, especially during a global health crisis.
- Potential Underestimation of Risks: The sheer number of reports indicating medical attention, emergency care, or hospitalization needed post-vaccination highlights a potential underestimation or underreporting of vaccine-related adverse events. This could imply that the initial safety and risk assessments communicated to the public might not have fully captured the breadth and depth of possible negative outcomes.
- Impact on Public Perception and Trust: The CDC’s handling of the vaccine injury data may contribute to public skepticism regarding vaccine safety and the overall trustworthiness of public health institutions. When the public perceives that health authorities may be withholding critical information, it can erode trust and affect individuals' willingness to follow public health guidance and participate in vaccination programs.
- Need for Robust Safety Monitoring: The situation underscores the necessity for robust, transparent, and ongoing safety monitoring of vaccines and other medical interventions. It highlights the importance of having systems like V-safe that can capture and analyze data effectively. However, such systems only prove their value when their findings are made promptly available to the public and are used to inform health policy and practice.
- Legal and Ethical Obligations: The CDC's reluctance to release the data until faced with legal challenges demonstrates a concerning gap between ethical obligations to inform and protect public health and the actual practices followed. It raises questions about the ethical standards governing the dissemination of health information and the accountability mechanisms in place for public health agencies.
While the CDC should be playing a vital role in safeguarding public health, these revelations call for a reassessment of its communication strategies, data transparency, and responsiveness to adverse event reporting. Restoring public trust requires a commitment to timely, complete, and transparent reporting of health data, ensuring that the public can make informed decisions based on comprehensive and accurate information.

Carl Riedel is an experienced writer and Open Source Intelligence (OSINT) specialist, known for insightful articles that illuminate underreported issues. Passionate about free speech, he expertly transforms public data into compelling narratives, influencing public discourse.